5 Easy and Essential Steps to Teaching Empirical Formula
Do you give your students steps to follow when working empirical formula problems? Students love steps to follow. It makes the information so much easier to process.
But, sometimes it is hard to come up with exactly the right steps, so I’ve laid them out for you. Just copy them down or pin this post so you can share them with your students.
Let’s get started.
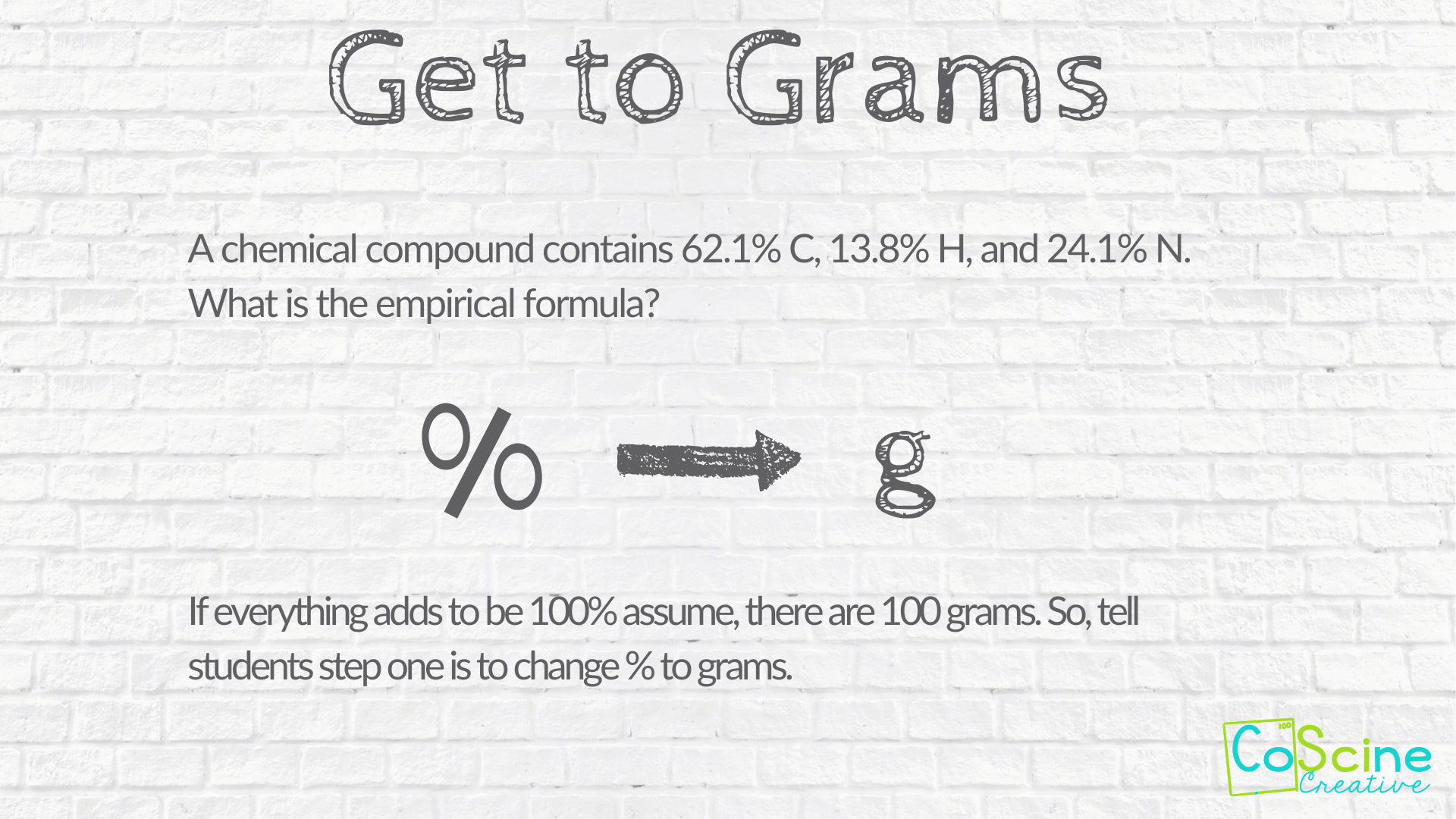
#1 Get to Grams
Tell students to convert percent to grams by dividing by 100. But be sure to write this step down as well as explain to students what you are doing as you do it.
When you begin to teach these problems to your students, start with problems that require them to convert percentages to grams as opposed to the problems that start with grams.
That way, in problems students don't have to convert to grams, they feel like they get to "skip" a step. If you teach it the other way around, students feel like you are giving them extra work.
#2 Set Up Converting to Moles
Tell students the second step is converting from grams to moles. To do that, they need to divide the grams by the atomic mass of the element.
Explain to students that they will have to look the number in the denominator up by finding it in the periodic table. Otherwise, they will ask you 164 times. In one day.
At this point, stop and ask if any student has a question.
#3 Divide by Atomic Mass
Students are often unsure of how far to copy down the decimal. Tell them to write the new number to three decimals (or however you prefer.).
Tell your students not to round until the end of the problem. That will keep all of your students at the same place with the similar numbers.
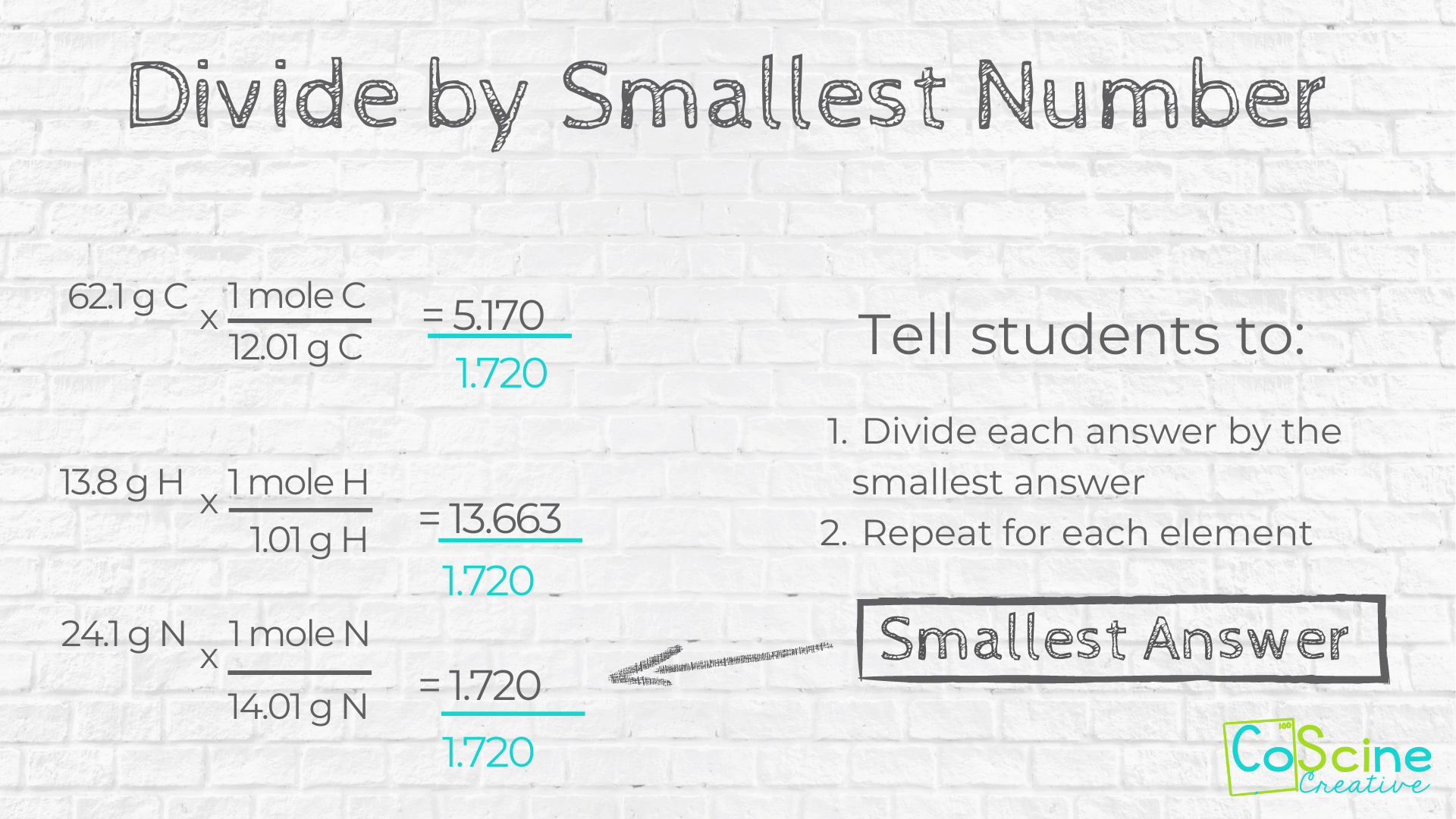
#4 Divide by Smallest #
Tell students at this point they will have 2 or 3 or 4 awkward looking numbers and that is ok. Tell them to pick the smallest awkward number and divide all the by that number.
For example, looking at 5.170, 13.663, and 1.720, 1.720 is the smallest number. Divide 5.170, 13.663, and 1.720 by the 1.720.
#5 Use those Numbers as Formula Subscripts
The last step should get students a whole number. That is the whole point of the division. Tell students that if they got 2.001 go with 2. Tell students we must have whole numbers.
You may also want to mention to your students if they end up with a 2.5 they need to either 1) check their work or 2) multiply all the numbers by 2 to make it even.
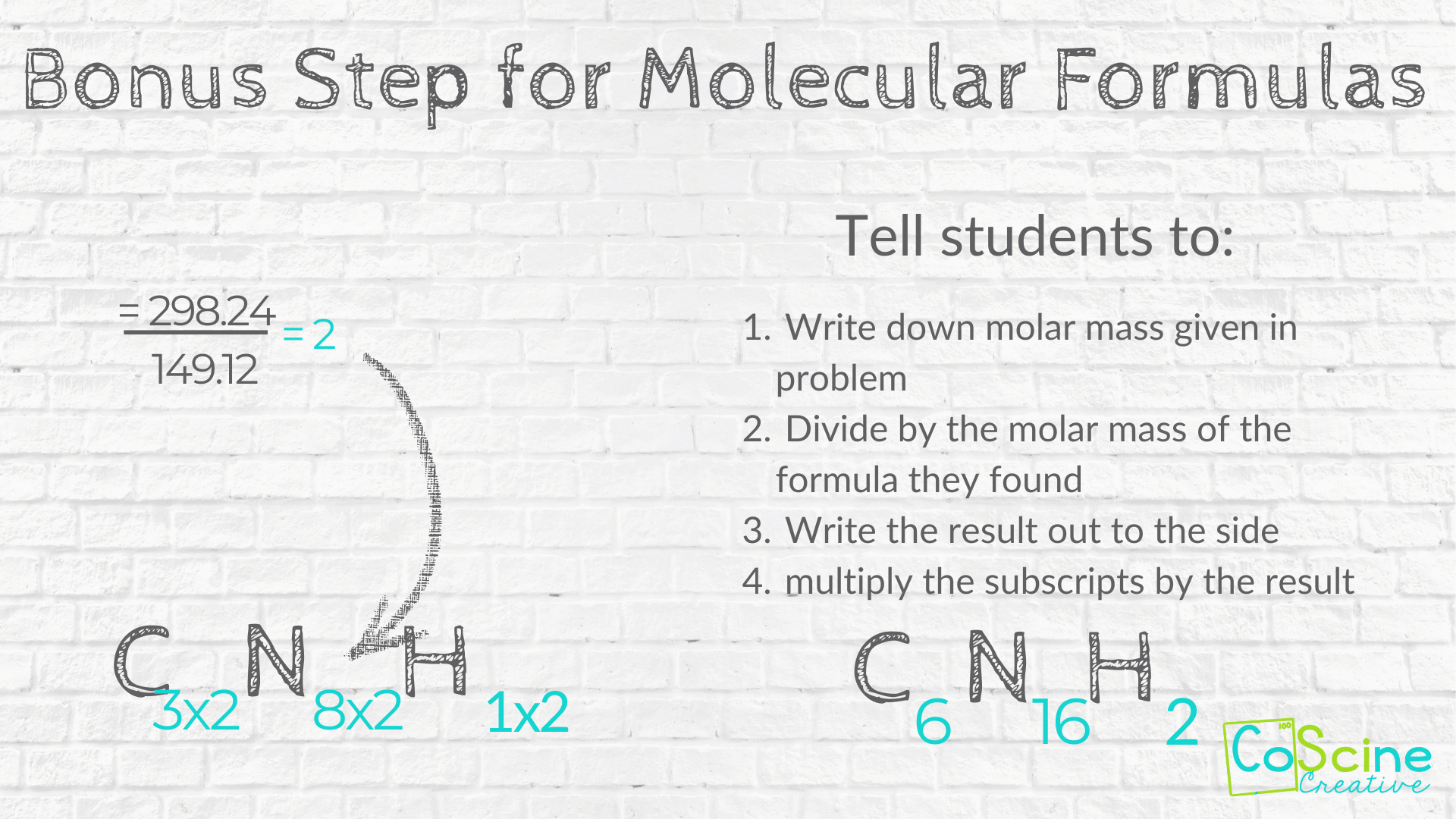
Bonus Step for Molecular Formulas
Tell students if they need to find the molecular formula, find the molar mass of their current formula, and divide by the molar mass given in the problem.
Your students are going to love that you gave them these steps!
When you put examples on the board, make sure you do one example where you do the work and write the steps right next to the work. Even better, color code the steps to the work.
If you liked these steps, you are going to love this illustrated guide to empirical formulas where you will make this process super easy for students to learn.