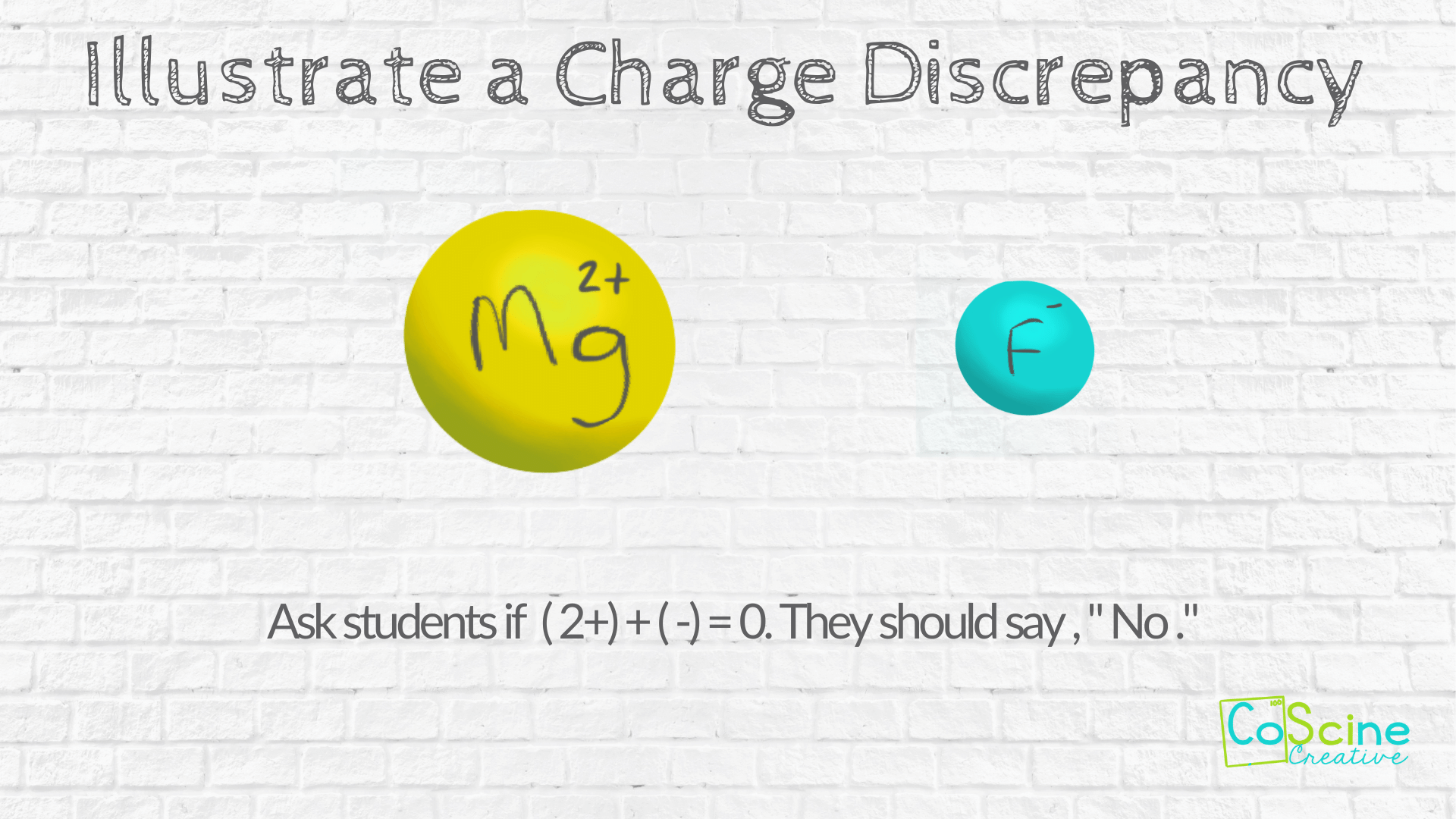A Visual Way to Teach Balancing Chemical Charges
If your students just aren’t getting the more mathematical approach (aka, crossing charges), maybe try a different tactic.
Illustrate for them WHY we balance charges.
#1 Show Them a Simple Molecule First
Put a simple chemical compound up that has a +1 to -1 charge ratio. Also, make sure it doesn’t contain a polyatomic ion. Something like NaCl, KCl, or NaF.
Write the symbols out, but also draw the elements as circles and write the charge inside the circle.
Ask them if (1+) +(1-) =0.
The students should say yes.
#2 Illustrate the Charge Discrepancy
Next put up two elements that do not have the same charge. Use Mg and F.
Draw Mg with the charge inside the circle and draw F with the charge inside the circle.
Ask your students if (2+) + (1-) = 0
(hint: It doesn’t)
#3 Ask Students How to Fix the Overall Charge
Ask your students how to fix the previous equation.
(2+) + (1-) = 0
Lead students to the idea that they would need two (-1) charges to balance those charges.
Show them that because of that, the formula for MgF would have to be MgF2, because it needs two negative 1 charges.
#4 Repeat with Two New Elements
Use lots of different example types when you teach this.
The last example showed what happened when the negative charge is too small. Show students an example or two where the positive charge is too small.
Something like Na₂O.
#5 Bonus Throw in a Polyatomic Ion
Once you feel like your students have the hang of it, I would say do at least 3-4 in-class problems, then add in the polyatomic ions(blog post!).
When you add in the polyatomic ions, start small like NaOH. Then move to something like Na₃PO₄.
Make sure that the last example you show is something like Mg₃(PO₄)₂. That way students are okay with polyatomic ions, but you aren’t throwing them in the deep end right away.
Put it into Action
Of course there are many ways to teach balancing charges, or crossing charges, in teaching students to write chemical formulas. I hope this helped you consider a new way to do things if you are looking to change things up a bit.
You can check out these related worksheets in my TpT store, CoScine.