How Do You Teach The Quantum Numbers of Carbon?
Are you teaching quantum numbers tomorrow and need a refresher?
Great! I’ve got you covered.
The best part is I'm going to make this easier than you ever thought possible. Which means your students are going to cheering your name at the end of class and running home telling their parents what an awesome chemistry teacher you are.
Well, maybe not, but you might inspire a future chemist.
Are you ready to see an application of the easy way to teach quantum numbers?
Abbreviated Electron Configuration
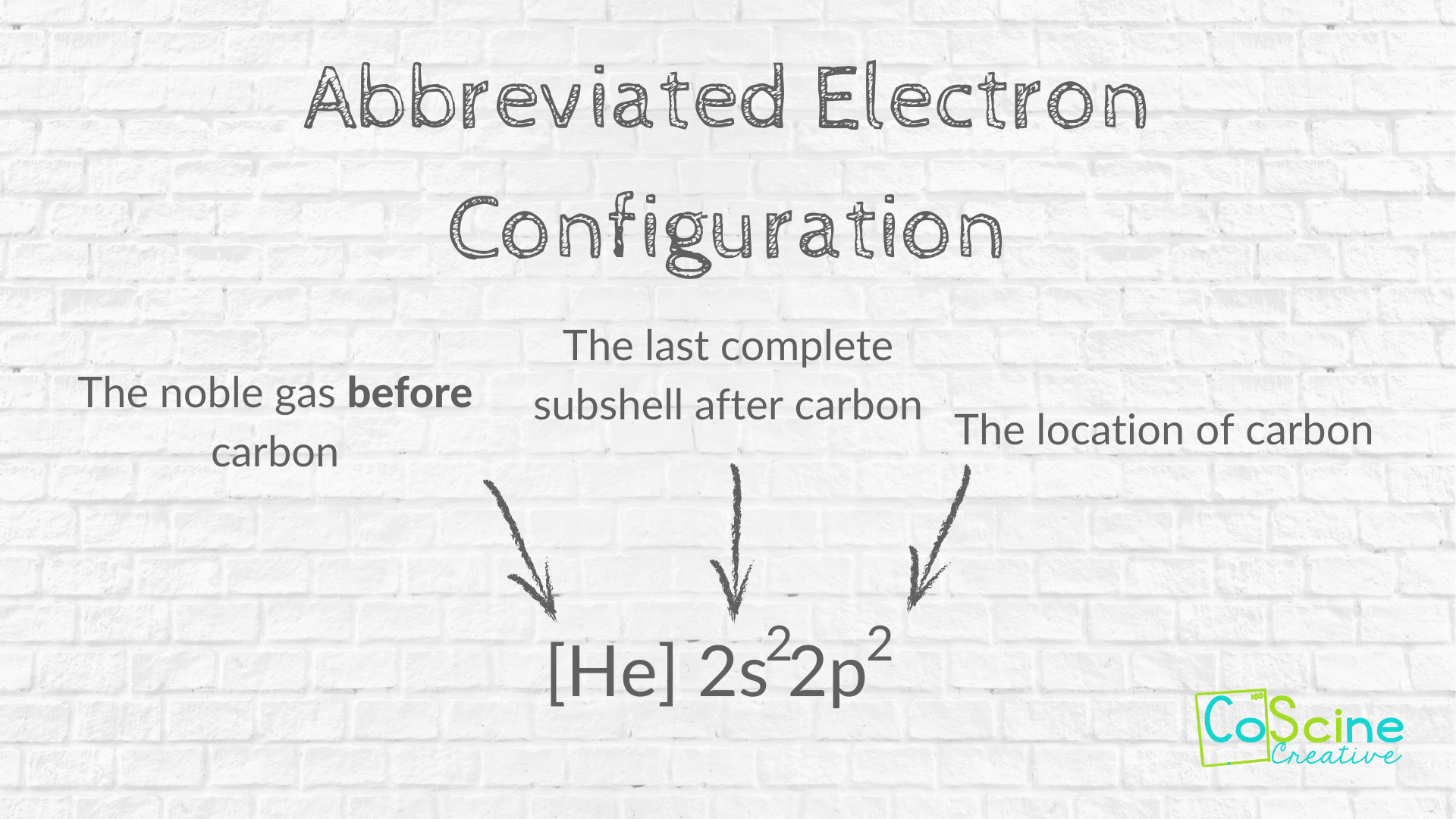
Step One: Have students write an abbreviated electron configuration.
For carbon, it will be [He] 2s² 2p².
If you aren't sure about how to teach electron configuration, read this post.
Abbreviated Orbital Diagram
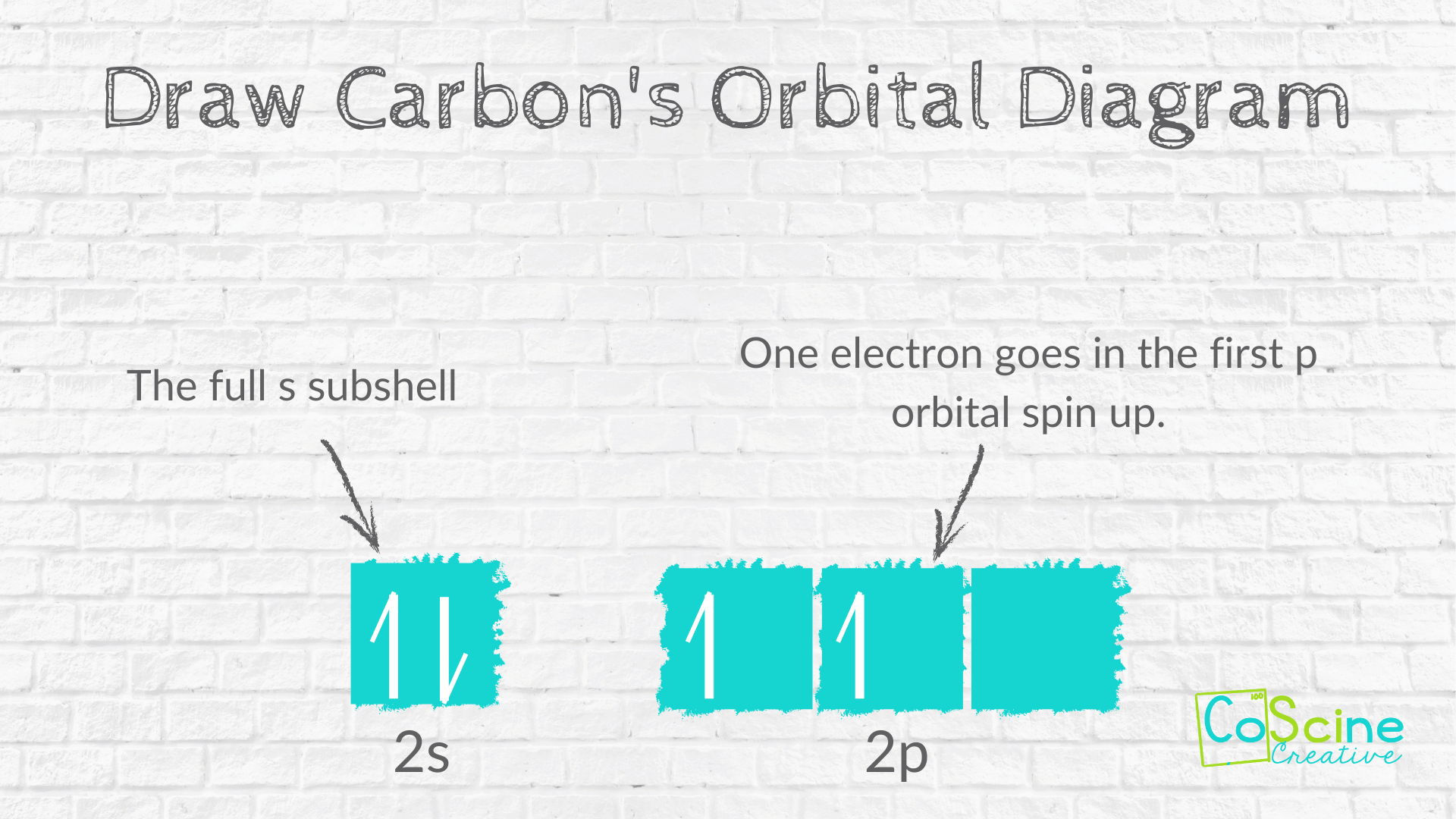
Step Two: Have students draw the abbreviated electron diagram for the valance electrons they wrote above. They should draw one box under the 2s² and 3 boxes under 2p².
Next, they should fill the first box with a spin up arrow, then a spin down arrow.
The p subshell has 2 electrons. According to Hund's Rule, students must put in electrons spin up until each box has an electron, then put in electrons spin down. So, the first two 2p boxes will have a spin up arrow.
Tell your students, “I know this seems like a lot of set up, but trust me it's 1000% worth it.”
The Quantum Numbers
Now comes the easy part! As long as your students set everything up right from the previous steps, the quantum numbers will be as simple as pointing to the number and writing it down.
That sounds like homework your students will actually complete!
How to Find "n" for Carbon
Step Three: Find the last coefficient you wrote.
For quantum number "n", have students look at the electron configuration. Ask them, "What was the last coefficient you wrote?"
(I often use the phrase “big number” so I don’t scare my students.)
Say to them, "That's right, it is that second big 2."
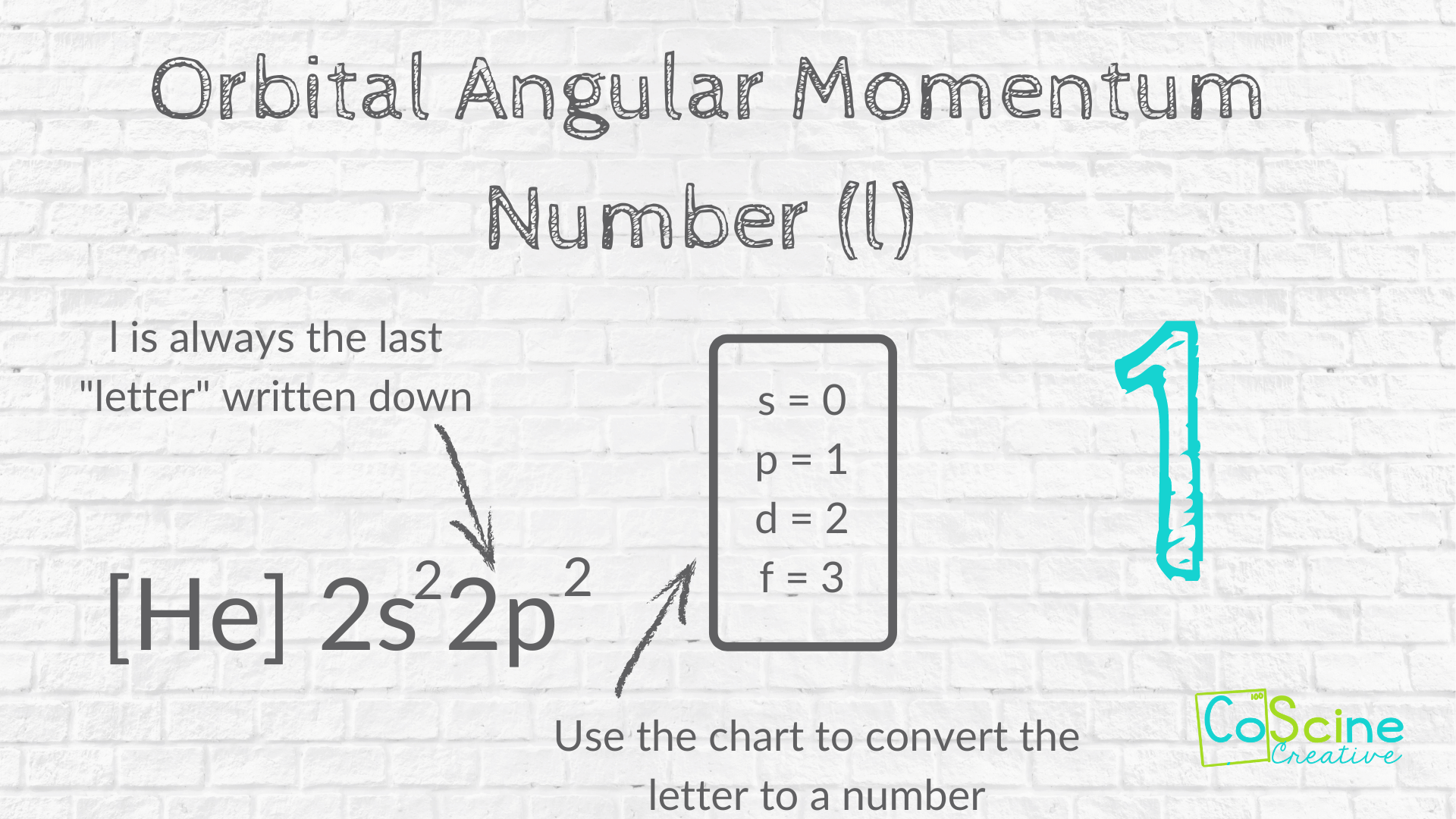
How to Find "l" for Carbon
Step Four: Find the last letter you wrote. Then use the chart to convert the letter to a number.
For quantum number "l", have students look at the electron configuration.
Ask them, "What was the last subshell you wrote?"
(I often use the phrase “letter” so I don’t scare my students.)
Say to students. "That's right, p. And p is coded into 1."
How to Find "ml" for Carbon
Step Five: Have students compare their orbital diagram to the chart. Then pull the number off the chart as the answer.
For quantum number "ml", have students look at the boxes they drew earlier and look at the chart I have next to mine here.
In the chart, the first box is the -1 position. The second box is the 0 position. The third box is the 1 position. You can find the teaching chart handout for your students here.
Ask them, "You can clearly see which position the last electron went in at, right?"
They should say, "The 0 position."
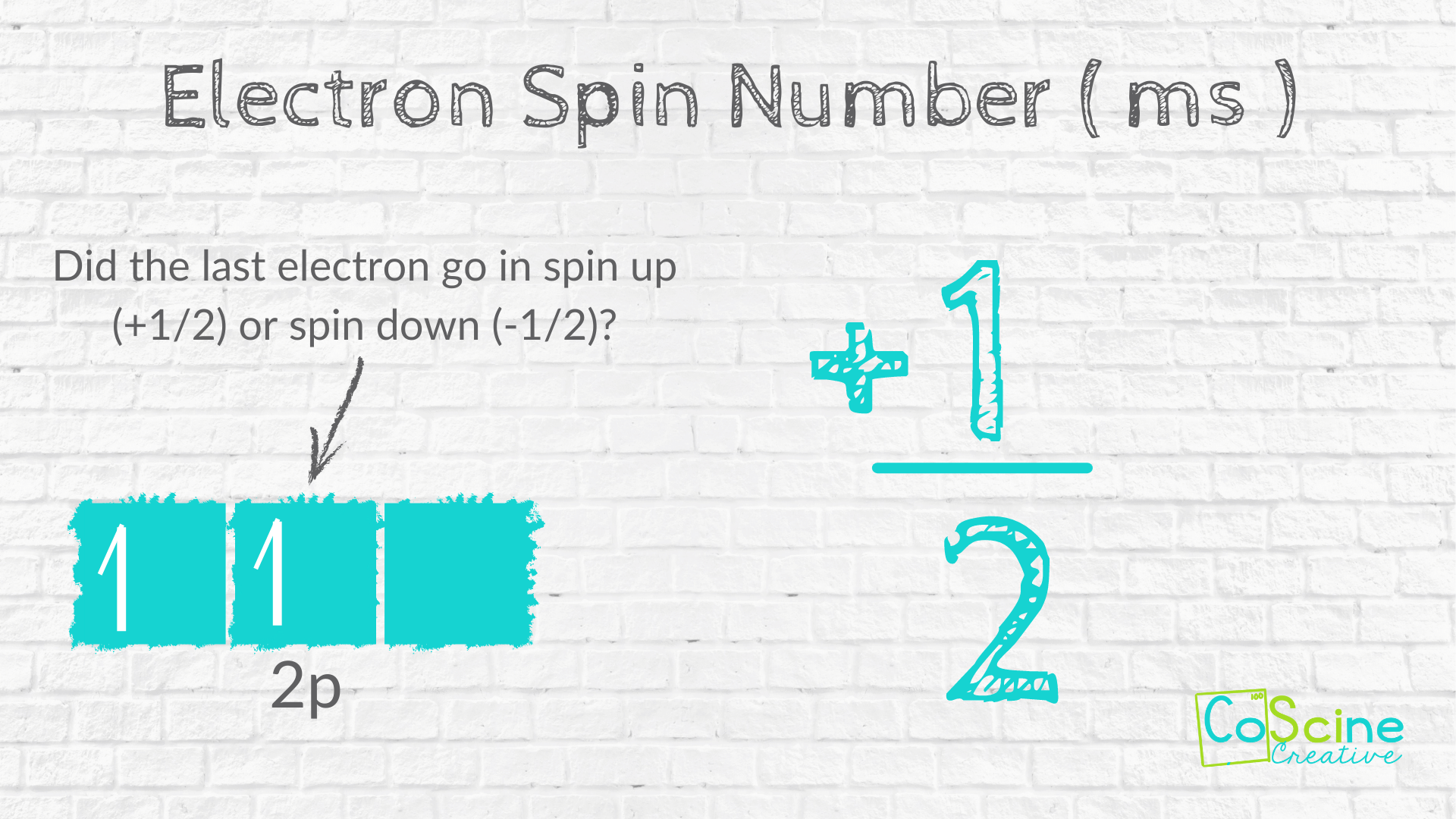
How to Find "ms" for Carbon
Step Six: Have students look at their orbital diagram to see if the last electron went in spin up or spin down.
For quantum number "ms", have students look at those boxes they drew earlier. Ask them, "Did the last electron go into the box spin up or spin down?"
Students should respond, "Spin up. So that is +1/2."
So the quantum numbers of carbon are 2, 1, 0, +1/2.
Download the Free Quantum Number Charts
If your students are anything like mine, they love step by step guidance that makes learning chemistry a less scary. Not to mention teaching it this way gives you more time because students learn faster. And you won’t have to repeat yourself 451 times in a day.
Read more about a fun demonstration for teaching quantum numbers here. (Spoiler alert: All you need is a coffee mug!)