How to Teach Atomic Number and Atomic Mass (So Students Remember it) (Copy)
Have you ever taught students how to find protons, neutrons, and electrons? Only to have them forget the next day? Nothing is more frustrating. You read the lesson. You searched Pinterest. You taught the lesson with a fun new worksheet. Only to have them remember nothing.
5 hours wasted. Should’ve watched Netflix like you wanted to.
It wasn't your fault. You needed a slightly different strategy that you didn't find on Pinterest that day.
You needed color coding and illustrations. (Yes, this is a lesson for high school students that's fun.)
Hit it, Liz!
Color Code Protons, Neutrons, and Electrons
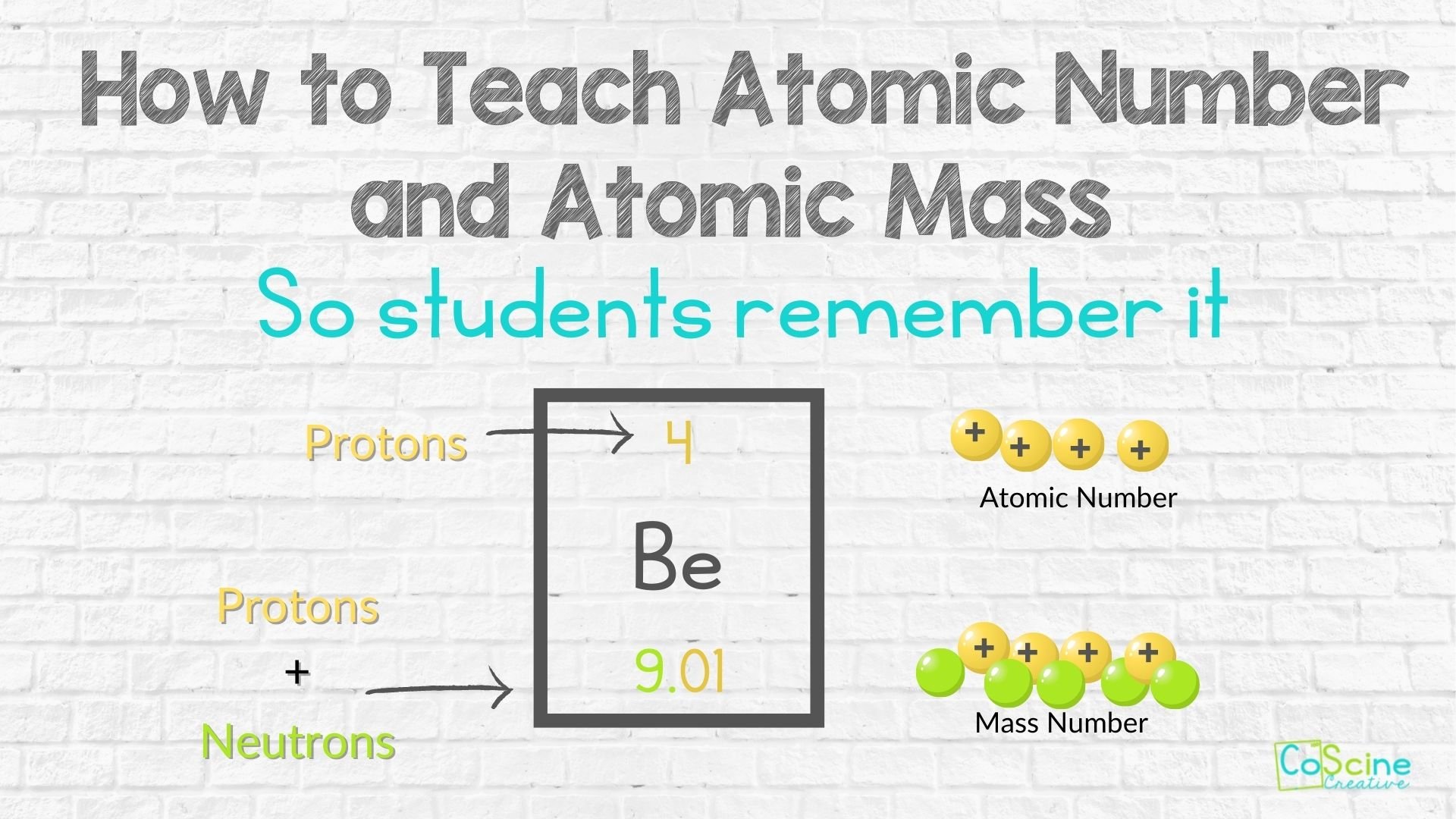
Are you familiar with CoScine’s color coding strategies? If so, you'll know that yellow is for protons. Why? Because it’s the color of sunshine and happiness. Anything positive throughout the curriculum is yellow.
Electrons are blue because they are sad, frustrated, and negatively charged.
Neutrons are neutral and get colored green . Why? Because blue and yellow make green.
Use these colors as the basis of your lesson to help your students gather building blocks for this and future lessons. One concept is going to build on another. (That's what’s missing in other textbooks and worksheets.)
Last, you’re going to use this information to build out memorable illustrations that will show students exactly why mass number and atomic number are different.
But, first, how can you show the atomic number?
Illustrate the Atomic Number
The atomic number is protons + neutrons right? Or is it just the protons? Or just the neutrons?
That's what students would normally say the next day after this lesson. But after teaching using this method they'll remember—and understand.
On your periodic table, point out beryllium. Then draw the element face on your board. Label the atomic number using a yellow marker. (Remember, protons are positive so they are yellow.)
Out to the side you are going to draw 4 yellow circles. Then put plus signs on them. These are your protons that the atomic number represents.
Congrats. You made an idea stick in brains that dump information faster than the speed of light.
Illustrate Mass Number
Draw an arrow to the mass number. Then label the word mass number in green and yellow. Explain that this is representing the protons and neutrons.
Not only have you labeled where the information comes from, but you have color coded it for students. And to top it off, you’ve drawn it out. Your students are going to think you are a genius.
More than that, you are chipping away at their fears that chemistry is going to be impossible. They are beginning to trust you.
Students now see the atomic number and mass number for the first time ever. You just made chemistry teacher history. (More importantly, you reduced the number of times you have to reexplain this.)
Use the Information
Now that students can see what the atomic number and mass number represent, you can show them how they are used.
Explain to students (and show them in a word equation or illustration) that the mass number - atomic number equals neutrons.
This will make finding protons, neutrons, and electrons so much easier on your students. You’ve brought the lesson to life and made it interesting.
If you’d like the free doodle notes to this lesson, check out the link in the podcast.