How to Teach the Difference in Protons, Neutrons and Electrons
Do you need a fun way to teach protons, neutrons, and electrons? Something new and different? Something that students’ will remember.
(You know how some lessons fall out of students’ brains as soon as they leave the classroom? This strategy changes that.)
Have you tried color coding and drawing subatomic particles? It’s not just for elementary students. The depth of understanding it brings to later topics that build on protons, neutrons, and electrons is unparalleled.
Color coding brings these particles to life.
But first, what does protons, neutrons, and electrons look like to students?
Do your students confuse protons, neutrons and electrons? Why is that? Look at it from a student point of view:
They look the same to students. (They picture these abstract little gray balls.).
They all sound the same since they end in “-tron or ton”.
So it makes sense students mix them up.
The student picture is fuzzy and bleak at best. The solution is to teach in color.
But not simply coloring. Using color as a tool, along with personifying each subatomic particle to give them character flaws that are memorable as well as entertaining.
What Color Should Protons be?
The CoScine Curriculum is based on color relating to charges. It makes remembering concepts easier. It makes processing new concepts effortless.
Yellow is for protons. Why? Because it’s the color of sunshine and happiness. Anything positive throughout the curriculum is yellow.
Draw a circle on the board. Color it yellow and give it a plus sign. Give it a smiley face. Or make it into a peppy cheerleader and make it radiate positivity.
If you are musically inclined play a song for your students like, “You are my sunshine.”
Whatever you do show a perky, positive personality on protons. Give them color to accentuate the personality you put on them.
Which brings us to electrons. Dun, dun, dunnnn.
What Color Should Electrons Be?
Electrons are blue because they are sad, frustrated, and negatively charged.
In short, they’re…Blue da ba dee da ba di…
Students may not know that song, but it will get the point across. This particle exudes negativity. Sometimes I make it cry negative tears or sweat negative signs when it’s angry.
You’ve laughed at the electrons pitching a fit if you’ve ever seen my doodle notes. Afterall, they have a touch of “short man syndrome” since they are so much smaller than protons and neutrons.
Neutrons have a different temperament. Whatever, bro.
Why are Neutrons Green?
Neutrons are neutral and get colored green. Why? Because blue and yellow make green.
That neutralization concept is found throughout the CoScine Curriculum.
Make sure you use color to accentuate how bored and ambivalent neutrons are. Neutrons are here. Because they were told to be here. But they’re bored.
They are what-ev-ah.
Use the Information
Use these colors as the basis of your lessons throughout the school year. It will help your students build a solid foundation for future lessons.
One concept is going to build on another. (That's one thing that’s missing in other textbooks and worksheets.)
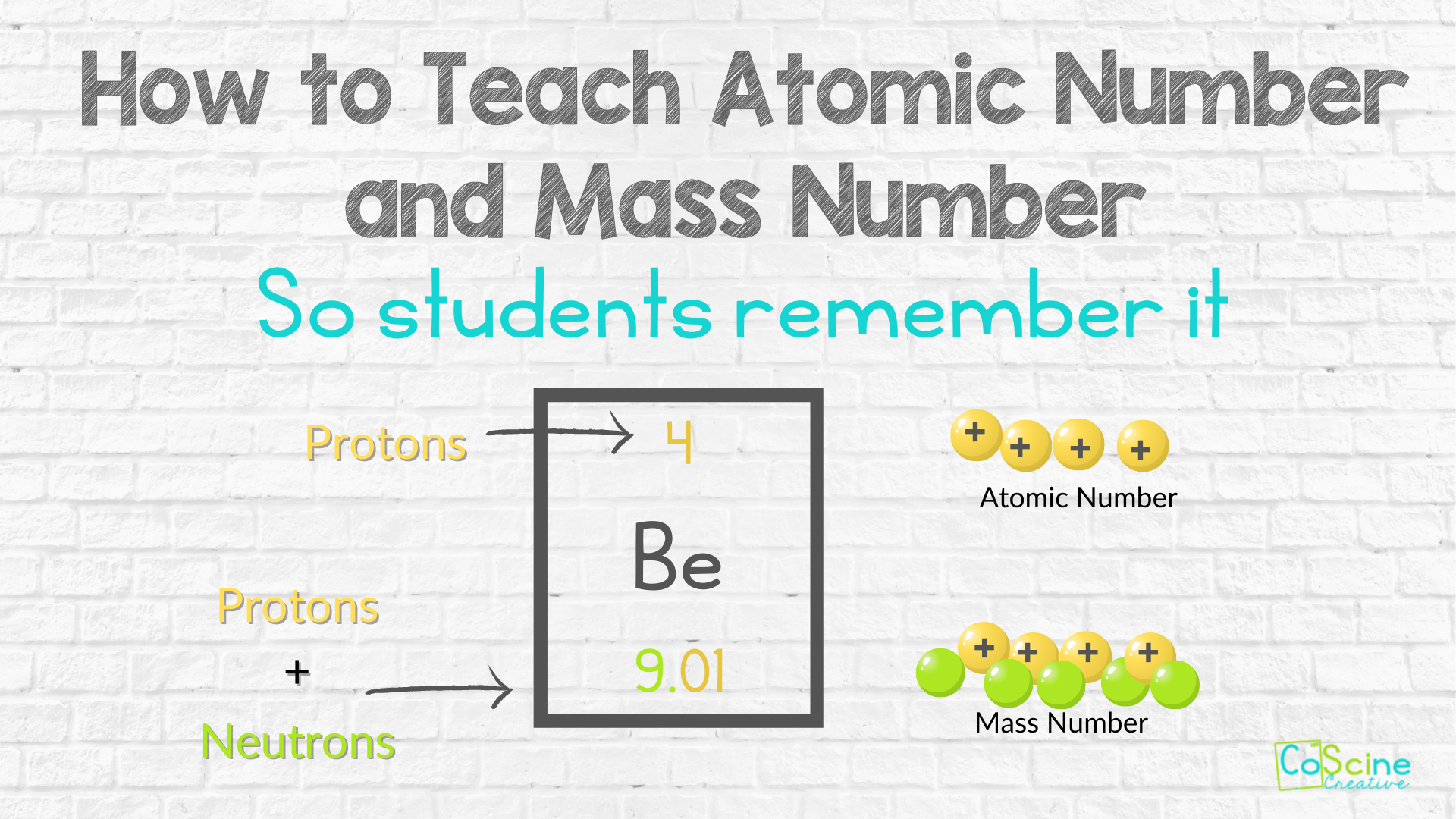
Congrats. You made protons, neutrons, and electrons memorable (and fun!). Now that students can see protons, neutrons, and electrons, imagine how easy it will be for them to understand atomic numbers and mass numbers using this technique.