Blog
Want an Easier Way to Teach Chemistry?
Learnings, teachings, tips & tricks for teachers to reference when you’ve heard “I don’t get it.” and “Where do those numbers come from?” 327 times a day. After implementing these ideas, you’ll get fewer mind numbing questions and see a lot more “Aha!” moments.
How to Teach Atomic Number and Atomic Mass (So Students Remember it) (Copy)
Is there a fun way to teach students how to find protons, neutrons, and electrons from atomic mass and mass numbers? Yes. In this post you’ll learn a few strategies for making this easy to learn.
How to Teach the Difference in Protons, Neutrons and Electrons
Is there a fun way to teach students how to find protons, neutrons, and electrons from atomic mass and mass numbers? Yes. In this post you’ll learn a few strategies for making this easy to learn.
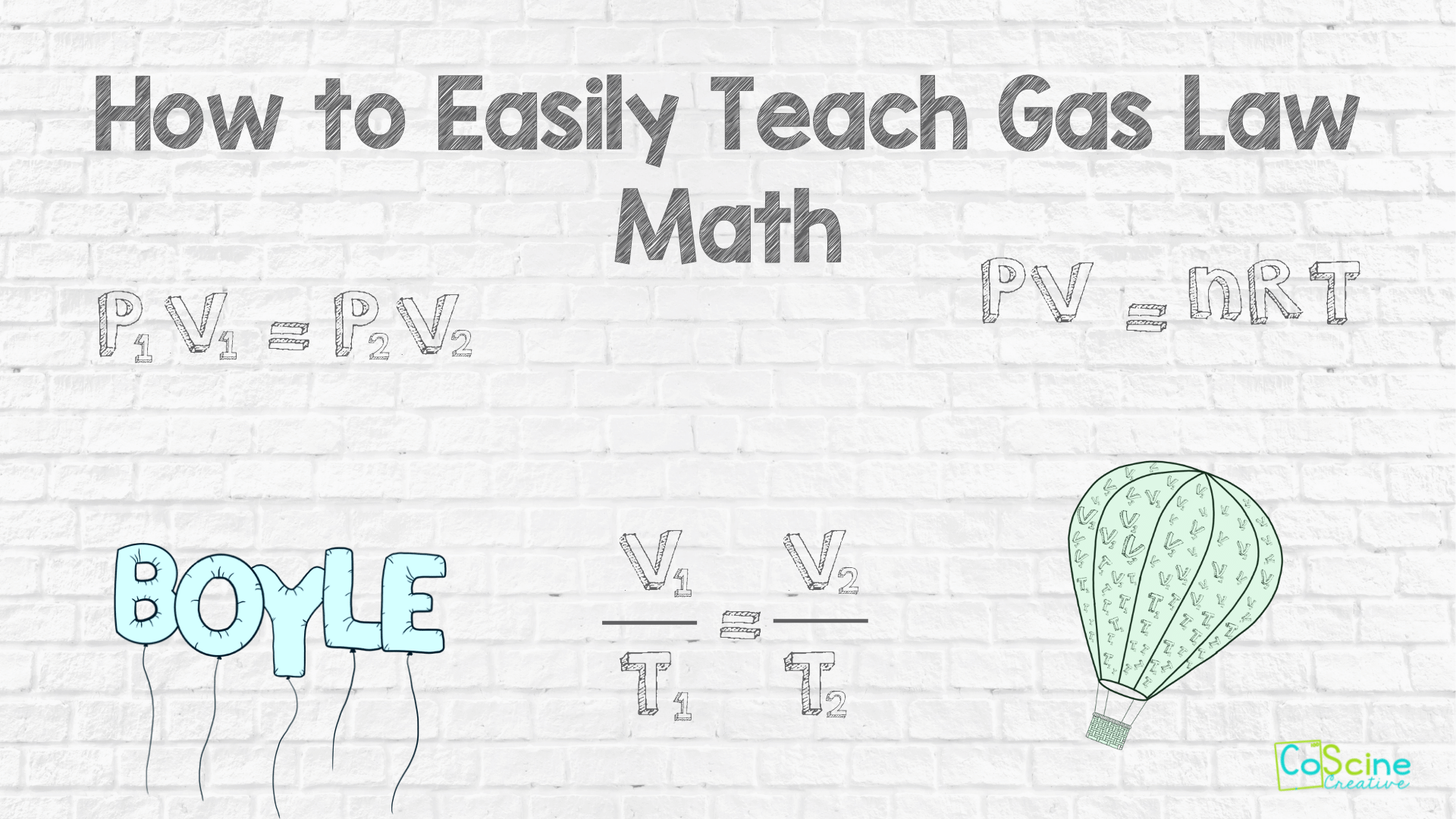
How to Teach Gas Law Math
Are your students setting gas law problems up correctly, but then making careless errors? Help them learn how to take notes on the math by color coding and writing the steps down neatly.
Are You Setting Your Students Up to Fail on Gas Laws?
Are you setting your students up to fail on gas laws? Is there a step that sometimes gets skipped that could make all the difference in your lesson? Knowing how to teach the math and explain the concepts are key.
How to Teach Chemistry (the Fun Way)
Before heading back to school, are you looking for ways to become a better chemistry teacher? The first half of this blog focuses on teaching strategies. The second part builds on those strategies in fun ways.
How to Teach Balancing Chemical Equations Visually
Are you looking for a fun and engaging way to teach balancing chemical equations? Use this method to help high school students learn easily the first time.
3 Sig Fig Illustrations That Make the Concept Stick
Are you looking for a fun and easy way to show your high school students the meaning of significant figures? These worksheets and illustrations will show you how to teach this lesson.
How Can Chemistry Doodle Notes Help Your Classroom?
Does using color and illustrations, help your students learn chemistry faster and retain more? What if I’m afraid doodle notes will bring down my classroom? The right set of doodle notes can make all the difference.
Easy Way to Teach Quantum Numbers
Teaching quantum numbers can be hard, but it doesn’t have to be. Toss aside your boring textbook and you’ll find an easy to teach and easy to learn method right here.
An Overview of Atom's First Chemistry
What is the atoms first method of teaching chemistry? Are you teaching the atoms first method of chemistry? You may not realize it, but the order you teach topics in is based on the method of chemistry you are teaching.
How Do You Teach The Quantum Numbers of Carbon?
Get simple, step by step instructions on how to teach students to find the quantum numbers of carbon. Students will love how fun and easy this method is.
13 Ways to Teach New Information in Chemistry
Are you stuck in a teaching rut? Do you want new ways to interact with students, deliver information, and show the lesson?
A Fun Way to Teach Quantum Numbers
Are you looking for a fun, no prep demonstration or activity for your high school chemistry students to learn the meaning of quantum numbers? Students will think you are a genius magician after this activity.
Students Show Understanding Through an Electron Configuration Game
After playing this game, students will be able to show you they understand how to write an electron configuration. Not only that, but high school chemistry students get lots of practice in during this activity.
5 Easy and Essential Steps to Teaching Empirical Formula
Students love when you give them steps to solving math problems in chemistry class. It also helps you see where they are struggling. Finding the empirical formula can be difficult for high school chemistry students, but these steps make it easy to teach and learn.
7 Mistakes Chemistry Teachers Make When Teaching Off PowerPoints
PowerPoint and Slides make teaching easier for us, but many of us haven’t been taught the correct way to teach off of slides. Are you making some of these mistakes in your high school chemistry class?
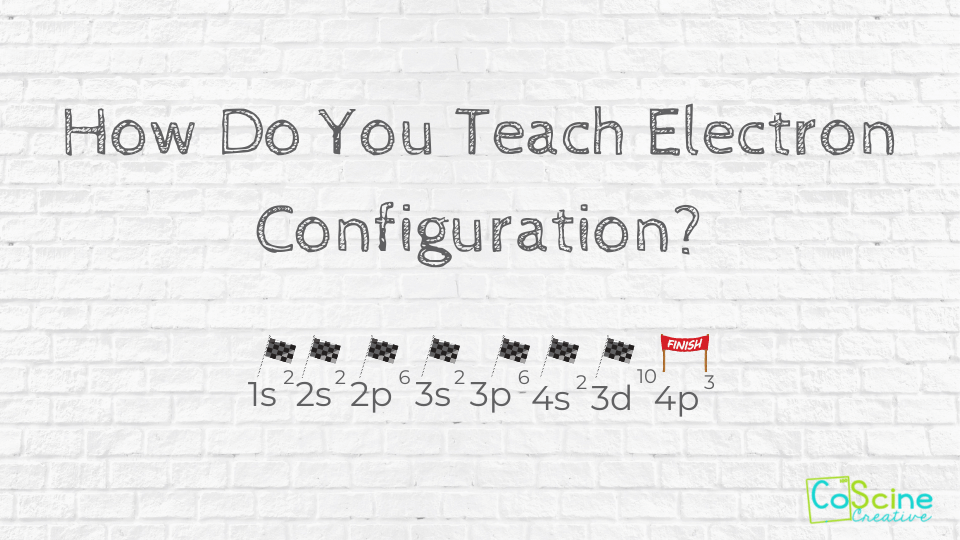
How Do You Teach Electron Configuration?
If you are looking for a fun analogy that keeps high school students interested while you teach them electron configuration, you’re in the right place. A race car will help students start off and keep going in the right direction.
5 Worksheets That Teach Writing Chemical Formulas for You
Writing chemical formulas can be dry, and boring. But these worksheets liven up your class with competition, color coding, and illustrations that will help your high school chemistry students have fun while learning.
Start your journey
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua.